REgulatory SCience INNOVATOR SUPPORT PROGRAMME 2025-2026
To support further the 69 companies who have taken part in the programme, as well as those who were unable to enrol, we are making most of the resources available to enhance access. We welcome your feedback on these resources and suggestions for additional topics you would find useful. Please get in touch with us at INFO@RADIANT-CERSI.ORG
-
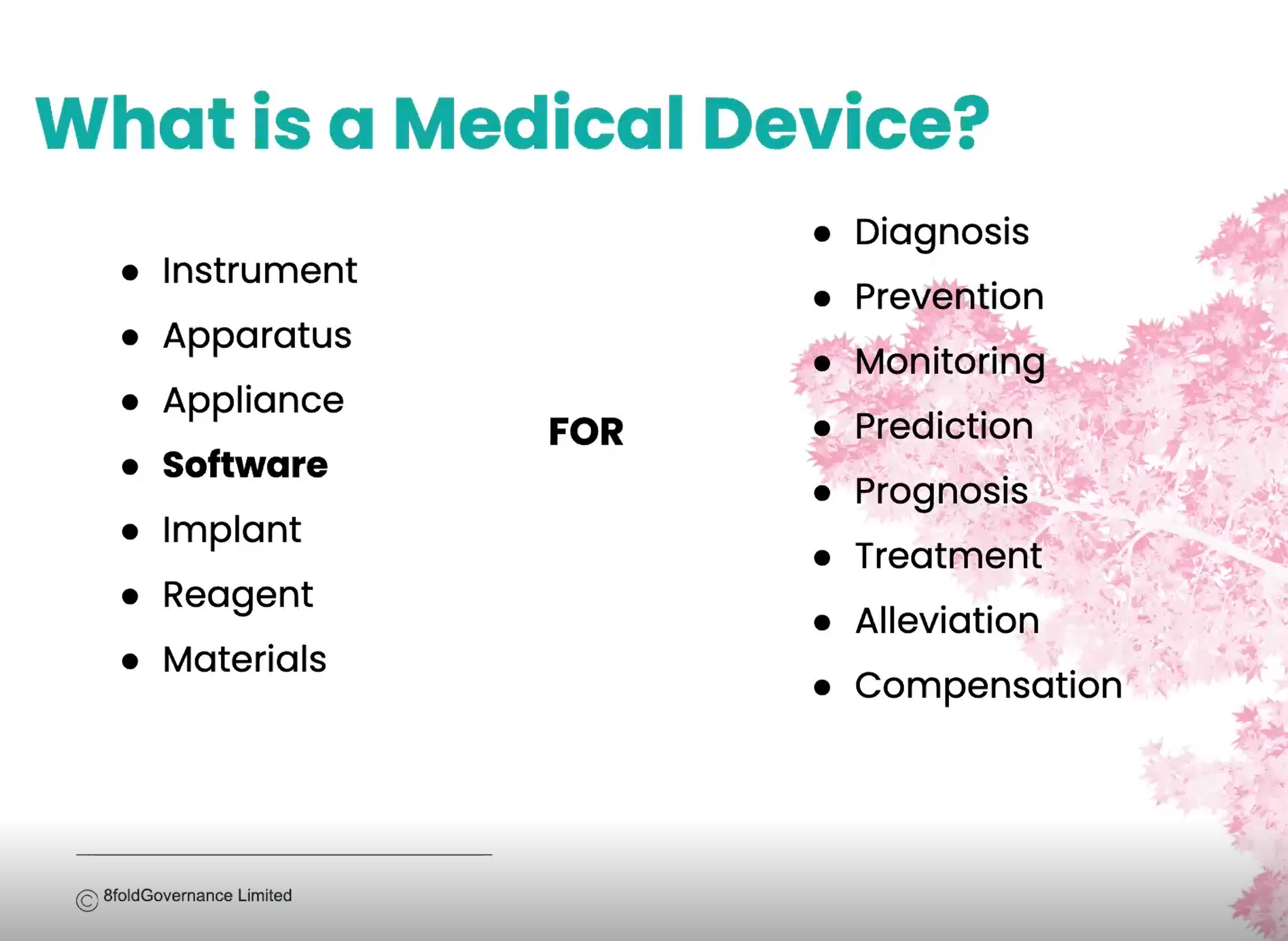
Introduction to Software / AI as a Medical Device
Introduction to Software / AI as Medical Device delivered by 8Fold Governance (https://8foldgovernance.com/)
-

Technical File 2 – Clinical Evaluation
Blum Health (https://blumtechgroup.com/) offer an overview of Clinical Evaluation of Medical Devices: Importance, Structure and Key Considerations, followed by an introduction to SPIRIT-AI and CONSORT-AI by Dr Tahmina Zebin, Brunel University of London
-

QMS and Post-market Surveillance
COMING SOON
-

Data Protection and Information security
COMING SOON
-

NHS regulation of digital tech and Apps, AI Framework
(DTAC, DCB0129, DCB0160)
COMING SOON
-

International standards and regulatory frameworks
COMING SOON
-

What’s different about AI as a Medical Device?
COMING SOON
Case Studies
See the real-world impact of the programme through the stories of other innovators




